Request a representative visit or G7 15 Day or G7 samples
A wide body of evidence substantiates the efficacy of Dexcom Continuous Glucose Monitoring (CGM) Systems for insulin-using patients with type 1 or type 2 diabetes (T1D/T2D).
Reduction of A1C by 1.0% for Patients with T1D*,1
Dexcom CGM System users on a multiple daily injections (MDI) insulin regimen showed an average A1C reduction of 1% after 24 weeks of regular use, compared to baseline. Additionally, 52% of subjects in the study had a ≥1% A1C reduction.1


Significant A1C Reduction for Patients with T2D
Patients with T2D on mealtime insulin achieved an average 1.4% A1C reduction after using Dexcom G6 for 12+ weeks†,2 allowing type 2 diabetes patients to better meet their target A1C goals.
Increased Time in Range
In a trial where Dexcom G6 powered the Tandem hybrid closed-loop system with Control-IQ, participants with type 1 diabetes increased their time in range (TIR) by an average of 2+ hours per day, allowing average blood glucose levels to stay in range longer.3


Improvement in Quality of Life
Patients with T2D on mealtime insulin experienced significant quality of life (QOL) score improvements after starting Dexcom G6.†,2
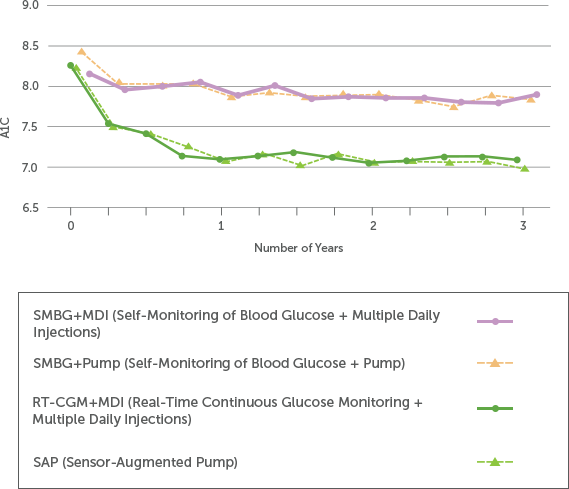
Patients Show Sustained A1C Reduction with Long-Term Real-Time CGM (RT-CGM) Use
A three-year, real-world clinical trial—the longest CGM outcome study to-date—reiterates the clinical impact of using real-time CGM (RT-CGM) systems like Dexcom G6.
Study participants with T1D using RT-CGM showed significantly reduced A1C levels, increased TIR (70-180 mg/dL), and decreased time below range (<70 mg/dL), independent of insulin delivery method.4
*Study conducted using the Dexcom G4® PLATINUM CGM System, which uses the same software as the Dexcom G5® Mobile CGM System. †Prior to participating in any study procedures, each subject was asked to voluntarily document their consent by signing an Institutional Review Board (IRB) approved informed consent form. ‡Compared to users who did not frequently engage with their CGM systems. Engagement in this real-world study was measured by the frequent use of Dexcom Clarity and the Dexcom Share/Follow features, as well as the customization of alerts.
1 Beck RW, et al. JAMA. 2017;317(4):371-378.
2 Gilbert TR, et al. Diabetes Technol Ther. 2021;23(S1):S35-S39.
3 Kovatchev B, et al. N Engl J Med. 2019;381(18):1707-17.
4 Šoupal J, et al. Diabetes Care. 2019;43(1):37-43.

