Request a representative visit or G7 15 Day or G7 samples
HypoDE Study: Dexcom CGM use resulted in a 72% Reduction in Hypoglycemic Events
HypoDE study shows that Dexcom CGM use significantly reduces hypoglycemia in MDI-treated patients with T1D at high risk for hypoglycemia.1
Background
Continuous glucose monitoring (CGM) clinical trials to date have largely excluded individuals with severe hypoglycemia (SH) or impaired awareness of hypoglycemia (IAH). The study examines effectiveness of Dexcom G5 Mobile CGM System* use in patients on multiple daily injections (MDI) insulin therapy with a history of SH or IAH.
Objective
Evaluate the effectiveness of CGM in reducing hypoglycemic events† among high-risk adults with type 1 diabetes treated with MDI.
Research Design/Methods
Randomized, controlled trial of 158 adults with type 1 diabetes.
- Randomized, controlled trial conducted in 12 diabetes practices throughout Germany
- 30-week study that included a 4-week baseline data collection period, a 22-week intervention phase, and a 4-week evaluation period (weeks 22-26) Adults with type 1 diabetes treated on MDI (n=149); randomized to CGM use (n=75) with the Dexcom G5 Mobile System,* or a control group using self-monitoring of blood glucose (SMBG) (n=74)
- History of IHA (Clarke score‡4) or recent severe hypoglycemic event
- Average baseline HbA1c of 58.0 mmol/L (7.5%)
Results
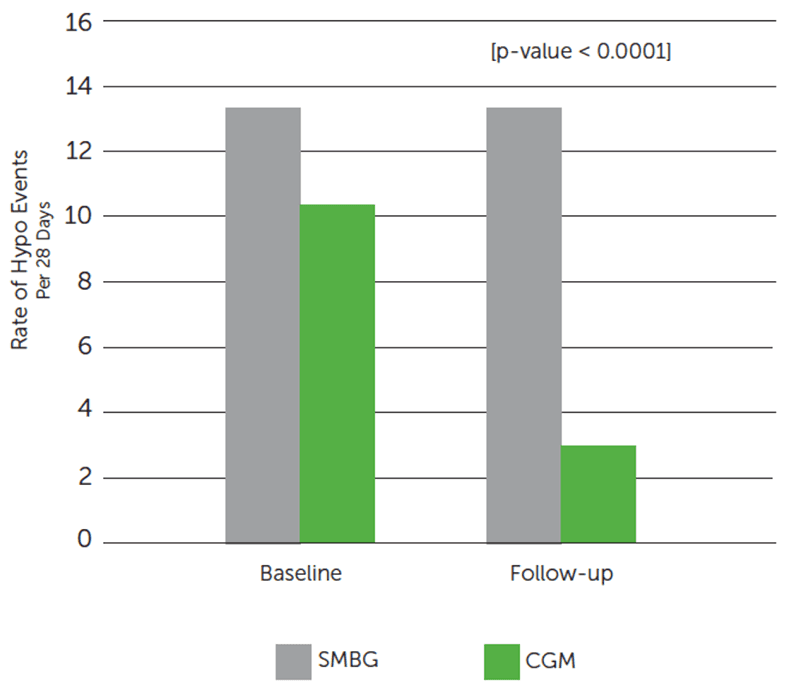
Dexcom CGM Use Reduced Exposure to Hypoglycemia.
Dexcom CGM group reduced the average number of hypo events by 72%§ from baseline.
SMBG group showed minimal reduction of hypo events (13.5 events at baseline; 13.2 events at follow-up).
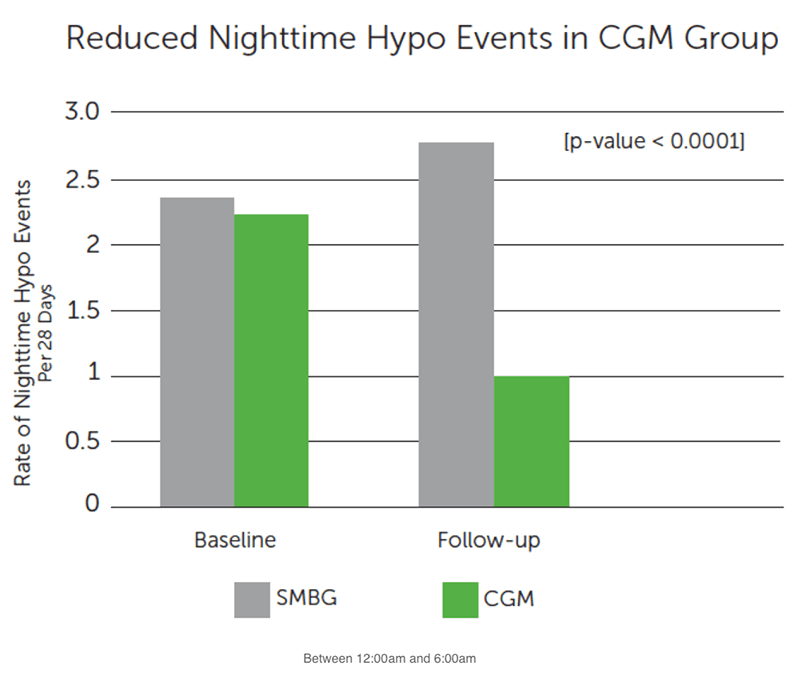
Secondary Hypoglycemia Outcomes:
- Reduced hypo events at night
- Significant reduction of hypoglycemia
- Reduced risk of severe hypo
Highlights:
- 72% reduction in average number of hypo events
- Decreased average number of nighttime hypo events by more than half
- Reduced risk of severe hypo
- Reduced hypo-related distress
- Reduced glycemic variability
*Baseline glucose data for all participants and the glucose data in the follow-up period for the SMBG group were collected using the Dexcom G4 PLATINUM CGM System, which uses the same software algorithm as the Dexcom G5 Mobile CGM System. Subjects in the CGM group used the Dexcom G5 Mobile in the treatment phase and the follow-up period. All CGM data collected in the baseline period and in the control group in the intervention period was blinded.
†Hypoglycemic event defined as glucose values ≤3.0 mmol/L (≤54 mg/dL) sustained for at least 20 minutes, preceded by a minimum of 30 minutes with glucose values >3.0 mmol/L (>54 mg/dL). Number of hypoglycemic events in baseline and follow-up phases were recorded for each patient and standardized to an incidence of hypo events per 28 days.
‡The Clarke score measures hypo awareness, based on a response to eight questions characterizing an individual's exposure to moderate and severe hypo events. Scores ≥4 indicate IHA.
§Adjusted for baseline differences.
1 Heinemann L, Freckmann G, Faber-Heinemann G, Stefania Guerra S, Ehrmann D, Waldenmaier D, Hermanns N. Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: Results of the multi-centre, randomised controlled HypoDE study. Lancet. 2018 Apr 7;391(10128):1367-1377.
Related Content
Topics: Pregnancy, Accuracy, Safety Research Spotlight: “Accuracy and Safety of Dexcom G7 CGM Use in Pregnancy Patients with Diabetes” Polsky S, et al. Diabetes Technol Ther. 2024;26(5):307-12...
Title: Long-Term Improvements in Glycemic Control with Dexcom CGM Use in Adults with Noninsulin-Treated Type 2 Diabetes Topics: Long-Term Outcomes, Type 2 Diabetes, Noninsulin-Treated Research...

